
Ask Indegene (Beta)
Hello, how can I help you today?
You may type your question or choose from the options below:
Thank you!
We'll be in touch. In the meantime, feel free to keep exploring!
04 Nov 2025
Executive Summary
Life sciences CRM landscape is at a pivotal inflection point. Veeva has announced the end of its long-standing partnership with Salesforce by September 2025, while Salesforce has responded with the launch of its Life Sciences Cloud (SFLC) in partnership with IQVIA. This development represents a decisive shift for pharmaceutical, biotech, and MedTech organizations that have long relied on Veeva CRM as their system of engagement.
This handbook provides a strategic roadmap for organizations considering CRM migration from Veeva Vault CRM to Salesforce Life Sciences Cloud. A full “rip and replace” approach is neither practical nor advisable. Instead, a hybrid CRM migration strategy is recommended, where Salesforce serves as the intelligent engagement hub for commercial, medical, and clinical workflows, while Veeva Vault continues as the system of record for regulated content, quality, and compliance.
Key risks to manage :
Compliance gaps arising from re-engineered workflows that must remain GxP-validated
Data harmonization complexity when unifying healthcare professional (HCP), healthcare organization (HCO), patient, and engagement records across platforms
User disruption if change management and phased rollouts are not carefully orchestrated
Quick-win opportunities :
Commercial acceleration through omnichannel engagement, call planning, and AI-driven insights
Unified data views enabled by Salesforce Data Cloud, reducing silos and ETL overhead
Productivity gains from Agentforce AI copilots that automate tasks, surface insights, and recommend compliant actions across roles
This paper is designed for CxOs, IT leaders, and Commercial and Medical executives evaluating their CRM strategy in response to these market shifts. It introduces Salesforce Life Sciences Cloud, outlines its four core pillars, and details the six migration streams that must be carefully orchestrated for a successful transition.
The objective is clear: to help organizations move confidently toward a modular, AI-enabled CRM future, while protecting the integrity of regulated systems and ensuring seamless continuity for field, medical, and patient-facing teams.
What is Salesforce Life Sciences Cloud?
Salesforce Life Sciences Cloud (SFLC) is an end-to-end intelligent engagement platform designed specifically for the life sciences industry, including pharmaceutical, biotech, and MedTech organizations. Built on Salesforce’s trusted, HIPAA-ready, and GxP-compliant foundation, SFLC unifies clinical, medical, and commercial operations. It enables organizations to harmonize data across disparate sources and deliver data-driven, compliant, and personalized experiences for both healthcare professionals and patients.
Powered by Agentforce, Salesforce’s digital labor platform, SFLC integrates AI, data, and industry expertise to automate routine tasks, orchestrate engagement, and accelerate decision-making across the life sciences value chain. For organizations planning a CRM migration to cloud, SFLC provides a scalable, future-ready foundation that aligns with best practices in Salesforce CRM implementation.
Through deep partnerships with organizations such as IQVIA and integrations with real-time data sources, SFLC helps life sciences companies break down silos, maintain regulatory compliance, and achieve faster time to value. It supports a hybrid CRM migration strategy, allowing enterprises to modernize engagement while retaining critical regulated systems. The result is a single, unified platform for the future of healthcare engagement.
Core Pillars of Salesforce Life Sciences Cloud
SFLC’s capabilities are organized into four core pillars.

1. Salesforce Platform: The Foundation
The Salesforce Platform provides essential capabilities such as workflow automation (Flow), secure data access, analytics, and omnichannel user interfaces. MuleSoft integration ensures seamless connectivity with other enterprise systems. Organizations can also build custom applications and AI-powered agents on this foundation.
2. Data Cloud: The Unification Layer
Data Cloud establishes a single source of truth for patients, HCPs, and HCOs. Its Zero Copy architecture, combined with Retrieval-Augmented Generation (RAG), ensures real-time, accurate, and integrated data across clinical, medical, and commercial workflows. This layer eliminates silos, enabling personalized engagement, advanced analytics, and insights-driven decision-making without the need for complex data replication or heavy ETL processes.
3. Life Sciences Cloud Modules: The Process Layer
This layer consists of pre-built, industry-specific workflows spanning the life sciences value chain. The modules include:
01
Clinical and R&D
Trial recruitment, participant recruitment, site selection, site management, and advanced therapy management
02
Patient Services
Patient support programs and patient registry management
03
Medical Affairs
MSL engagement and medical information management
04
Commercial Operations
Field rep enablement, key account management, meetings and events, and contact center operations
4. Agentforce AI: The Experience Layer
Agentforce functions as an intelligent assistant that overlays all workflows. Role-based AI agents support MSLs, field reps, patient services teams, and other stakeholders by generating insights, automating routine tasks, drafting communications, and recommending the next-best actions.
Indegene Insight : Organizations can begin with the Platform, Data Cloud, and Core Commercial Modules today, and progressively expand into medical, clinical, and AI-driven workflows as Salesforce delivers roadmap releases in 2026.
Roadmap and Pricing Editions of Salesforce Life Sciences Cloud
Salesforce released its Life Sciences Cloud roadmap earlier this year, outlining the phased rollout of features over the next 18 months.
1
September 2025 (General Availability)
Most core commercial features will be available, with initial capabilities focused on foundational account management, engagement, reporting, strategic planning, and task tracking
2
February 2026
Enhancements will expand to include order management, medical insights, and key opinion leader (KOL) management, further strengthening medical and operational efficiency
3
July 2026 and beyond
The roadmap emphasizes advanced collaboration, enhanced field and key account management (KAM) agent functionalities, pre-call planning, automated reporting, and analytics, enabling a fully integrated, cross-functional CRM ecosystem
The figure below maps the planned features against the Salesforce roadmap for clarity.

FAQ: Does Life Sciences Cloud replace existing clinical, medical, and patient systems, or complement them?
Life Sciences Cloud is intended as a strategic complement rather than a one-to-one replacement of validated systems such as CTMS, EDC, PV, or Veeva Vault. Salesforce positions LSC as an orchestration and engagement platform, supporting a hybrid CRM migration strategy for life sciences companies.
Salesforce has already included patient services and certain clinical engagement features in general availability (such as recruitment and enrollment primitives, inventory management, and patient services tooling). However, deeper capabilities in clinical automation, customer engagement, and Agentforce remain on the roadmap and will be delivered in staged releases.
For regulated source-of-truth functions, the recommended pattern is to integrate LSC for engagement and orchestration while maintaining validated systems as the authoritative records. In practice, this approach allows organizations to benefit from modern Salesforce CRM implementation while continuing to rely on CTMS, EDC, PV, and Vault as validated systems of record.
On the commercial side, LSC is particularly strong. It leverages Salesforce’s proven CRM capabilities, including omnichannel engagement, rep and MSL enablement, HCP 360, analytics, and automation. For enterprises exploring Salesforce Service Cloud implementation or broader commercial transformation, LSC helps close the loop between clinical evidence and commercial action.
Salesforce Life Sciences Cloud Editions and Pricing
Salesforce Life Sciences Cloud is offered in four editions, each designed to meet different maturity levels across commercial, medical, and clinical teams. The table below reflects publicly listed prices as of August 2025, following Salesforce’s 6% price adjustment across Enterprise and Unlimited editions.

Note: Prices are per user per month and reflect Salesforce’s public listings.
Key Pricing Considerations
Migration costs are not included in license fees
Data Cloud usage is credit-based; scaling requires additional credits
Clarify which modules are currently GA versus on the roadmap, and whether future features can be locked in at today's pricing
Explore multi-year, enterprise-wide, or global discounts (benchmarks are typically 20–40%)
Confirm whether MuleSoft connectors and OneKey/partner integrations are included or separately licensed
Understand how Flex Credits are priced, whether baseline credits are bundled, and if unused credits can roll over
Sample “All-In” Scenario for a Mid-Sized Pharma
Team Size: 100 field reps
Edition: Unlimited ($555 per user per month) → ~$55,500 per month (~$666,000 per year)
Add-ons: Salesforce Maps, Sales Enablement, Medical Email Generation (~$120,000 per year combined)
AI Usage: Agentforce pilots with ~2M Flex Credits (~$150,000 per year)
Estimated All-In License Cost: ~$950,000–$1.0M annually (before discounts)
Note: This estimate excludes migration, validation, training, and integration costs, which require a domain-centric technology partner familiar with modern workflows, compliance, and molecule-to-market journeys.
Indegene Insight : Most organizations should begin with Unlimited Edition, which balances functionality (automation, analytics, and collaboration) with cost. Enterprise Edition is suitable for smaller markets or pilot rollouts, while Agentforce Editions are best adopted once AI governance, data readiness, and change management practices are firmly in place.
How Salesforce Life Sciences Cloud Differs from Veeva Vault
Several key architectural and strategic differences distinguish Salesforce Life Sciences Cloud from the traditional Veeva ecosystem.
Platform vs. Suite Approach
Veeva’s ecosystem is built around specialized applications (Vault CRM, Vault PromoMats, Vault RIM, etc.), each focused on a specific domain. SFLC, by contrast, is built as one extensible, unified platform. All SFLC modules share the same data model and core services, reducing data fragmentation and eliminating duplicate integrations. For example, an HCP record enriched in Salesforce Data Cloud is available simultaneously across Sales, Medical, and Clinical modules.
AI-Native Architecture
SFLC seamlessly incorporates AI from the outset. Agentforce embeds real-time recommendations, summaries, and automation directly into user workflows. For instance, instead of manually preparing call notes, a sales representative can request “key points from last year’s interactions with Dr. Smith” and instantly receive a digest. Veeva, by contrast, is adding AI features incrementally (such as AI-certified content review partnerships). Salesforce also offers built-in predictive analytics through Einstein AI, supporting use cases like sales forecasting and patient risk scoring, which Veeva lacks natively.
Unified Data Layer
SFLC’s Data Cloud provides a single source of truth for HCPs, HCOs, patients, trials, and outcomes. This enables integrated profiles that span CRM data, trial data, and third-party sources such as claims or EHRs. Veeva’s architecture traditionally requires separate integrations between Vault CRM and Vault RIM/Quality systems. In SFLC, clinical and commercial data coexist, enabling smoother coordination. Notably, Salesforce continues to partner with H1 and IQVIA for HCP reference data and announced a OneKey connector for HCP data in late 2025.
Ecosystem and Integration
SFLC benefits from Salesforce’s broad ecosystem. Native integrations with Tableau CRM, Slack, MuleSoft, and other Salesforce clouds provide access to a vast partner network and reusable components. Marketing Cloud and Slack, for example, extend automation and collaboration capabilities with minimal integration effort. By contrast, Veeva environments often require custom integrations—even between Veeva CRM and Veeva Vault—for use cases such as sample compliance or multichannel marketing.
Compliance and Security Flexibility
Both Salesforce and Veeva are built for regulated life sciences. Veeva Vault CRM is GxP-validated out of the box, with FDA/EMA audit-ready processes. Salesforce takes a more flexible approach. Using Salesforce Shield (audit trails, data masking, etc.) and third-party validation frameworks such as Sware, organizations can design custom compliant workflows. In other words, Salesforce provides a configurable compliance toolbox, while Veeva delivers fixed, pre-validated templates.
Read more about how Veeva Vault and Salesforce Life Sciences Cloud compare side by side in our detailed analysis
Strategic consideration: The choice between SFLC and Veeva often comes down to breadth versus depth. Veeva emphasizes deeper, purpose-built life sciences functionality, particularly around content management and samples. Salesforce, in turn, emphasizes a broader, AI-driven platform with a future-ready roadmap. The reality today is that Salesforce Life Sciences Cloud can replace most core CRM functions and provides a vision for integrated engagement. However, Veeva Vault continues to set the standard for regulated content, quality, and R&D systems, especially in clinical and medical domains.
Hybrid Path: The Near-Term Future of Life Sciences Cloud Adoption
Veeva currently holds the majority of the market share in life sciences technology solutions. Migrating to SFLC requires careful consideration of which systems to retain and which to replace, even when starting with Salesforce as the commercial CRM platform.
The table below summarizes commercial-use-case Veeva solutions, their SFLC counterparts based on announced roadmaps, and where gaps remain:

Replace
SFLC plans to provide equivalent functionality by late 2025 or later

Retain
The Veeva system is expected to remain the primary solution for now
This hybrid approach allows organizations to leverage SFLC for intelligent engagement and orchestration, while continuing to rely on Veeva for regulated, validated, or specialized processes.
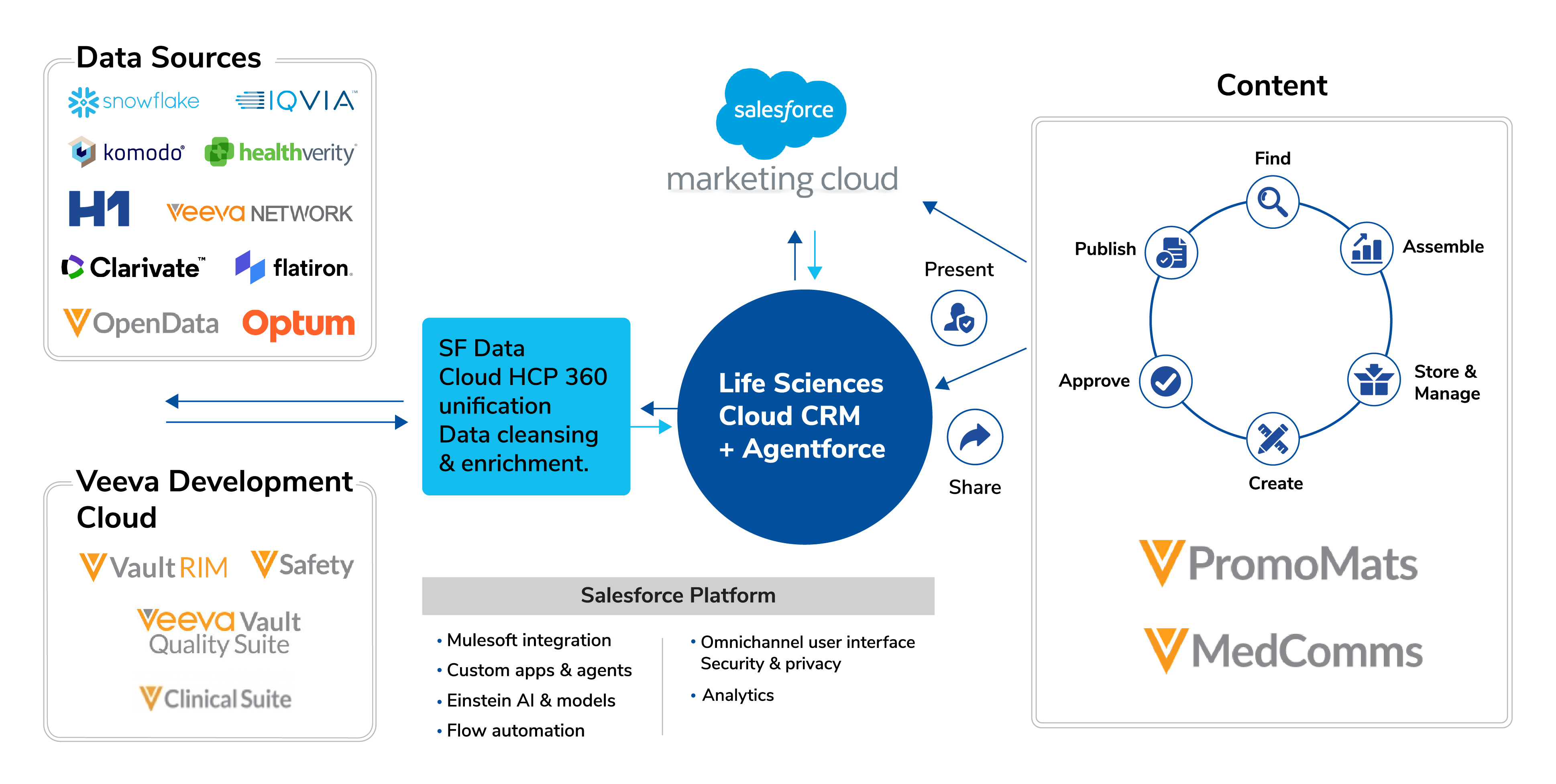

Mapping the CRM Stakeholder Journey in a Hybrid Environment
In a hybrid healthcare ecosystem, understanding how CRM stakeholders interact across multiple touchpoints is critical for delivering a seamless experience. This journey maps how stakeholders, including Key Account Management, Marketing, Data, Digital, and IT teams, engage with CRM systems at each stage of their workflow.
By visualizing these interactions, organizations can identify opportunities to:
Optimize engagement across commercial, medical, and clinical teams
Streamline processes and reduce operational friction
Ensure critical insights are accessible across both digital and field channels
The following journey highlights the flow of interactions, showing where stakeholders’ responsibilities overlap, where collaboration is essential, and where hybrid tools enable a more connected and efficient experience.
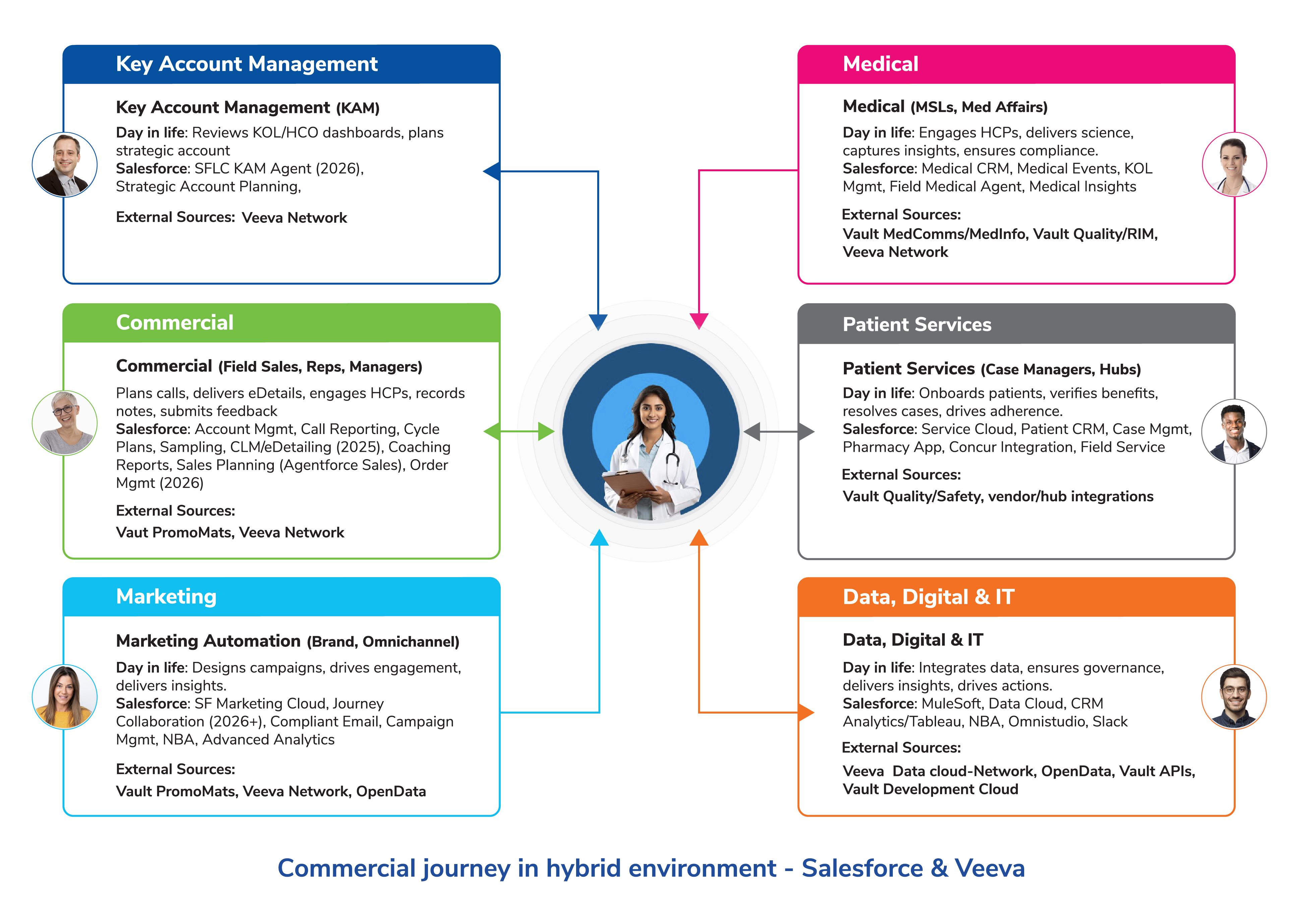
6 Key Migration Workstreams for Pharma Teams
Migrating from Veeva CRM to Salesforce Life Sciences Cloud (SFLC) is not a simple lift-and-shift. It requires careful planning, staged execution, and attention to data architecture, business processes, and regulatory compliance. The main workstreams global pharma companies should consider include:
1. Data Migration
Data is the foundation and getting it right is critical. A clear approach is needed for mastering data, transactional records, and historical content.
Master Data : HCP and HCO data from Veeva CRM and Veeva Network must be harmonized into Salesforce Data Cloud. Organizations may also enrich this data with third-party sources such as IQVIA OneKey. Salesforce Data Cloud does not replace Veeva Network as the golden source; it functions as a unified engagement layer consolidating account hierarchies, affiliations, territory alignments, and consent preferences.
Transactional Data : Sales calls, CLM/eDetailing interactions, sample disbursements, compliant emails, event participation, and medical inquiries must migrate to SFLC to maintain analytics continuity and compliance. KOL interactions and congress engagements are particularly important for Medical Affairs.
Historical Records : Most organizations do not migrate all Vault content. Vault often remains the system of record, with links or metadata surfaced in Salesforce. Legacy CRM call notes, cycle plans, and adverse event records should be archived while remaining query-able for compliance.
Mapping Challenge : Veeva includes many life-sciences-specific custom objects. Mapping these to Salesforce standard objects and Data Cloud entities requires careful field-level design. Typically, a combination of ETL pipelines for bulk or clinical data and API-based integrations for real-time commercial and medical data works best. Salesforce’s zero-copy approach reduces duplication, but upstream systems may require preparation.
2. Integrations
Hybrid coexistence is often unavoidable in the near term.
Upstream Clinical & Regulatory : If systems like Veeva Vault RIM, eTMF, CTMS, or Safety remain, bidirectional integrations are needed so study and safety data flows into Salesforce for commercial and medical teams. ETL can handle structured trial data, while APIs can manage real-time alerts.
Downstream Commercial & Patient Services: SFLC should integrate with Marketing Cloud, Health Cloud, payer/HUB data, benefits verification platforms, and specialty pharmacies. Agentforce relies heavily on these external data streams for insights.
Third-Party Data: IQVIA OneKey, Komodo, and formulary databases enrich Salesforce Data Cloud and enable segmentation, targeting, and trial recruitment.
Analytics : Dashboards must be rebuilt in Tableau and Einstein, combining Salesforce data with residual Veeva data until migration is complete.
Agentforce AI Integration
Salesforce introduces role-specific AI agents that transform engagement for HCPs, patients, investigators, and internal teams. Their effectiveness depends on trusted, regulated data from Veeva Vault and partner systems. A hybrid strategy ensures Salesforce agents drive engagement while integrations provide compliant, validated data.

3. Content Migration
Content drives daily user interactions, so accurate migration is highly visible. Organizations must convert content into SFLC structure while ensuring rendering is consistent.
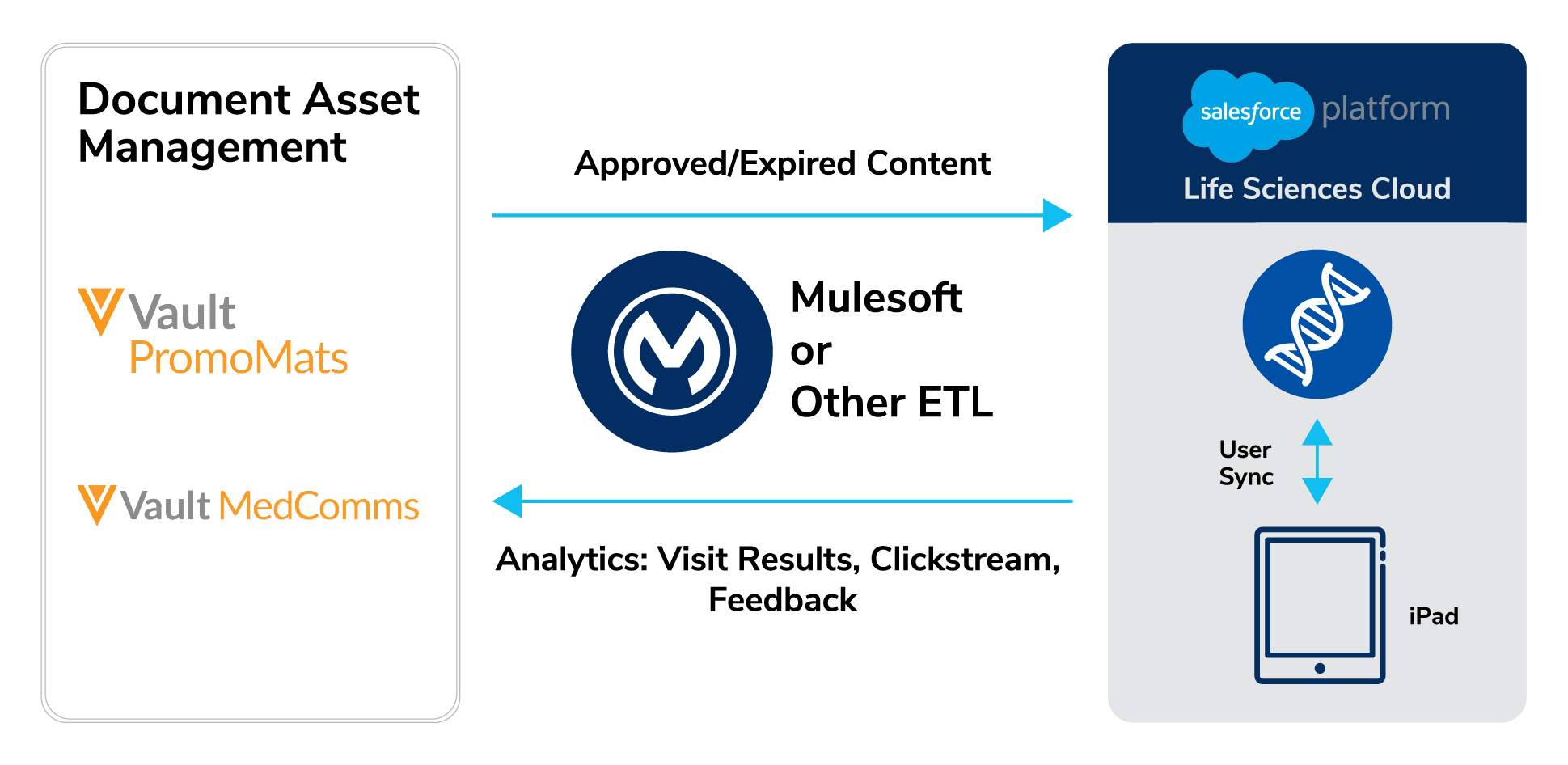
Key Content Types:
CLM and eDetailing Assets: Presentations, HTML5 assets, and interactive modules must migrate into Salesforce CLM/eDetailing. Re-authoring may be required.
Approved Email Templates: Veeva Approved Emails must be recreated in Salesforce's compliant email framework, preserving approvals and opt-out controls.
PromoMats Content: Vault PromoMats typically remains the system of record. Salesforce can access content via API to search and retrieve approved materials.
MLR Workflows: If Vault is retired, MLR processes must be re-implemented in Salesforce via Flow or partner add-ons, meeting Part 11 requirements.
Indegene Insight: Salesforce provides accelerators for content export, structure conversion, taxonomy mapping, function mapping, and format conversion. Some manual effort and partner support may be required for complex unmapped functions, fields, and pixelated functional validation to ensure no MLR approvals are required.
4. Process Re-Engineering
Systems migration alone is not enough; business processes must adapt to Salesforce design and roadmap.
Field Force: Rebuild pre-call planning, sampling, and activity capture in Salesforce Lightning and mobile apps. Offline workflows will gradually mature in Salesforce Field Agent apps by 2026.
Medical Affairs: Inquiry management and KOL engagement will transition to SFLC over time. Medical Inquiry agents will release in 2025, with KOL and medical event workflows in 2026.
Commercial Operations: Territory planning (Veeva Align) will move to Salesforce Territory Management or hybrid models. Dashboards should be rebuilt in Tableau or Einstein. Marketing orchestration moves to Marketing Cloud and Journey Builder, supported by Agentforce.
Compliance: Regulated processes like sampling, adverse event capture, and audit trails must be validated in Salesforce using Salesforce Shield and Trust Layer to achieve GxP and Part 11 compliance.
5. User Migration and Change Management
Migration is also about people.
Profiles & Roles: Map Veeva role hierarchies to Salesforce profiles, maintaining clear separation of medical and commercial roles. Security must comply with privacy and promotional regulations.
Training & Adoption: Users familiar with Veeva iPad require retraining on Salesforce Lightning and Field Agent apps. Early pilots showcasing AI-generated call prep and compliant email drafting boost adoption.
Phased Rollout: A dual-run period between 2026 and 2027 is realistic. Commercial teams may migrate first, with medical workflows remaining in Veeva until Salesforce capabilities mature.
6. Validation & Compliance
Regulatory approval is essential.
GxP Validation: Every workflow, from sampling to adverse event reporting, must be formally validated. Partnering with experienced consultancies is advisable.
Audit Trail Preservation: Vault audit logs cannot be migrated to Salesforce. Organizations must retain Vault or archive logs in a compliant storage solution. Salesforce Shield ensures future CRM records remain auditable.
Regulatory Sign-Off: Quality, Legal, and Compliance teams must re-approve workflows before production release. Without this, migrated processes cannot go live.
Best Practices for Global Multi-Brand CRM Migration from Veeva to Salesforce
Successfully migrating from Veeva CRM to Salesforce Life Sciences Cloud across multiple brands and regions requires strategic planning and execution. The following best practices have proven effective:

Secure Executive Sponsorship and Governance
Establish strong global leadership alignment supported by regional leaders. Form a global migration steering committee including IT, Commercial, Medical, and Quality functions, with regional representation. Define processes that must be standardized globally, such as compliance and reporting, while allowing local markets to adapt where appropriate.

Adopt a Phased, Value-Driven Rollout
Avoid a big-bang migration. Start with pilots in one brand or region, deliver measurable value such as faster reporting or cleaner HCP data, and expand gradually. Tie each phase to business outcomes and leverage early wins to build momentum.

Prioritize Data and Process Mapping
Treat data as the foundation. Document Veeva processes across all regions, perform global data profiling, and build mapping tables. Validate global standards through regional pilots to ensure consistency and accuracy.

Leverage Experienced Partners
Engage technology partners with global life sciences expertise. Use accelerators such as pre-built connectors, validation scripts, or migration frameworks to simplify HCP, sampling, and compliance migrations.

Invest in Training and Change Management
Begin enablement early. Provide sandbox access, multilingual training, and role-specific Trailhead modules. Implement a train-the-trainer model to develop local champions, and maintain regional helpdesks supported by a global playbook.

Maintain Compliance Vigilance
Involve Quality and regulatory teams from the start. Align global and regional compliance requirements, maintain validation documentation, and conduct mock audits. Utilize Salesforce audit trails and history tracking for transparency.

Communicate Transparently and Often
Share milestones, prepare markets for upcoming changes, and acknowledge regional variations, such as sampling rules. Clear communication about risks and timelines builds confidence across the organization.
Future-Proofing CRM in Pharma
Migrating from Veeva Vault CRM to Salesforce Life Sciences Cloud is a strategic initiative to future-proof an organization’s CRM and engagement platform. The unified, AI-enhanced Salesforce platform enables integrated data and smarter workflows across commercial, medical, and clinical domains.
This transition should follow a well-defined CRM migration strategy, not a single cutover. By identifying which Veeva capabilities to replace, such as sales force automation and digital engagement, and which to retain, including Vault for regulated content and quality, organizations can achieve the best of both worlds.
Key success factors include strong executive sponsorship, phased rollout, rigorous validation, and clear Salesforce data migration best practices. Costs and risks are significant; analysts note that CRM migration to cloud platforms like Salesforce can be “long, expensive, and risky” if not executed properly. These risks can be mitigated with structured Salesforce CRM implementation approaches and experienced partners.
For many enterprises, choosing the right Salesforce Service Cloud implementation pathway is just as critical as adoption of new engagement capabilities. Done right, it transforms not only technology but also ways of working.
At Indegene, we help global pharma and biotech companies navigate this complex transition, ensuring that CRM modernization not only meets today’s business needs but also positions teams for innovation and sustainable growth in the future.
Talk to us to learn more.
Let's Partner to Commercialize with Confidence

