The life sciences industry is experiencing significant transformation, driven by the growing need for seamless data integration, strict regulatory compliance, and improved operational efficiency. To tackle complex challenges—ranging from content marketing automation to workflow optimization and multi-channel communication—organizations are increasingly leveraging advanced technologies.
At the heart of this evolution is a unified automation model, which serves as a game-changer. It enables seamless integrations, leverages business rules engines, and utilizes low-code/no-code configurations to deliver measurable results. By automating repetitive tasks and simplifying complex workflows, this approach not only enhances efficiency but also drives better compliance, agility, and scalability.
Changing Markets and Evolving Regulations
Recent regulatory developments underscore the importance of transparency and accuracy in life sciences marketing and promotions. For instance:
- In 2021, the FDA issued guidelines emphasizing the accuracy of information used in promotions on social media and other digital platforms to prevent the dissemination of misleading content to patients and HCPs.
- Similarly, the EMA introduced a 2020 bill to enhance the transparency and traceability of medical device promotions throughout their lifecycle.
These regulations compel life sciences organizations to adopt ethical and accurate practices for the promotion and marketing of prescription drugs, medical devices, and other healthcare products. Non-compliance risks not only legal repercussions but also reputational damage, further underscoring the need for enterprise integration—seamlessly connecting compliance frameworks, data management systems, and marketing platforms to ensure transparency, accuracy, and adherence to regulatory standards.
Challenges

Driving Compliance and Efficiency with Enterprise Integration
Enterprise integration has become a transformative solution for organizations striving to meet regulatory demands while enhancing operational efficiency. Its benefits span across multiple dimensions:
- Boosting productivity: By automating workflows and eliminating repetitive tasks, enterprise integration technologies enable employees to focus on high-value strategic initiatives. According to SAP research, organizations leveraging integration have reported a 20-30% increase in productivity.
- Reducing errors: Manual data entry is often fraught with errors that compromise decision-making. Integrated systems significantly minimize human error leading to improved data quality and more reliable operations.
- Cutting operational costs: Streamlined processes and reduced IT complexity translate into tangible savings. This allows resources to be allocated more effectively to innovation and growth.
Emerging Trends in Life Sciences Data Integration
In life sciences digital transformation, data integration is evolving to support the following trends in reshaping how organizations manage data to drive innovation and regulatory compliance.
1. Content Factory Integration
Content factory integration focuses on creating a cohesive ecosystem by connecting content authoring platforms with content management systems like Veeva Vault PromoMats (VVPM) and further linking them to content distribution tools such as Salesforce Marketing Cloud (SFMC), Veeva CRM or CMS like Drupal, Sitecore, AEM and others. Middleware solutions like Workato, Azure Services, MuleSoft, and Informatica facilitate seamless data exchange, enabling efficient content lifecycle management and streamlining workflows.
Key Features:
- Automated data synchronization: Ensures seamless transfer of data between authoring tools and VVPM, as well as between VVPM and SFMC/CRM platforms.
- Real-time Integrations: Provides real-time integration and API management to support smooth communication across systems.
- Secure, scalable frameworks: Guarantees compliance with regulatory requirements through robust infrastructure.
Benefits:
- Compliance assurance: Maintains adherence to regulatory guidelines at every stage of the content lifecycle.
- Improved collaboration: Facilitates seamless teamwork across cross-functional teams working on approved content.
2. Business Rules Engine
Business rules engines are powerful systems that apply pre-defined logic to automate decisions, enforce compliance, and streamline workflows. When integrated with platforms like Workato, MuleSoft, or SnapLogic, they enable even greater potential by automating data-driven actions across interconnected systems.
Key Features:
- Centralized rule management: Enables consistent decision-making across diverse use cases by maintaining all rules in a single repository.
- Workflow automation in life sciences: Executes actions across systems using conditional triggers, enhancing operational efficiency.
- Low-code configuration: Allows dynamic rule updates to adapt to evolving regulations and business conditions with minimal technical effort.
Benefits:
- Consistency across processes: Ensures workflows adhere to defined rules, minimizing errors and maintaining quality standards.
- Increased productivity: Automates repetitive tasks, enabling teams to focus on strategic, high-value activities.
3. Low-Code/No-Code Integration
Low-code/no-code platforms are revolutionizing technology development by enabling business users to create and integrate applications without requiring extensive coding expertise. The emergence of large language models (LLMs) has further enhanced low-code and no-code capabilities, enabling non-technical users to build and integrate advanced solutions with greater ease.
Key Features:
- Drag-and-drop interface: Simplifies workflow automation and integration setup with minimal coding.
- Pre-built connectors: Enables seamless integration with CRM , CMS, DAM, and other enterprise systems.
- Customizable business logic: Allows users to configure rules and workflows tailored to their specific needs.
- Scalability and flexibility: Supports rapid adaptation to regulatory changes and business growth.
Benefits:
- Rapid deployment: Speeds up the creation and deployment of integrations and applications, reducing time-to-market.
- Cost-efficiency: Eliminates the need for large, specialized development teams, cutting down operational costs.
- User empowerment: Enables business users in pharma to build and customize solutions to meet evolving needs with minimal technical support.
4. Agentic AI in Enterprise Integration
Agentic AI is redefining business operations by seamlessly integrating into enterprise systems to drive efficiency and enable smarter decision-making. Unlike traditional AI, which focuses on automating routine tasks, Agentic AI operates autonomously, addressing complex challenges and aligning with strategic business goals to deliver transformative outcomes.
Key Features:
- Context-aware automation: Agentic AI continuously learns from data patterns and operational workflows to make intelligent decisions.
- Real-time data processing: Ensures instant analysis and execution of business actions without delays.
- Adaptive learning models: Evolves over time by refining decision-making processes based on new data inputs.
- Seamless system integration: Connects with existing enterprise platforms like Veeva Vault, OneTrust, and CRM portals for enhanced efficiency.
Benefits:
- Autonomous decision-making: Makes real-time, independent decisions without requiring human intervention.
- Competitive advantage: Helps businesses stay ahead in a data-driven world by leveraging advanced AI capabilities.
- Streamlined business processes: Automates and optimizes complex workflows, such as content approval processes.
Key Use Cases:
- Integrated Martech ecosystems: Seamlessly connect CRM, CMS, DAM and other platforms with minimal technical complexity, enabling efficient collaboration and data management.
- Streamlined workflows: Automating content management processes within platforms like Veeva Vault.
- Data synchronization: Automatically updates data across key martech platforms based on pre-set criteria.
- Targeted data routing: Filters and routes customer data to the appropriate marketing channels based on defined business rules.
- Personalized engagements: Delivering tailored recommendations through CRM portals to enhance customer interactions.
- Automated review and approval workflows: Streamlines the transfer of promotional content from authoring tools to VVPM for review and approval.
- Content distribution across platforms: Automates the movement of approved promotional content from VVPM to SFMC or CRM for execution.
- Real-time campaign management: Enables multi-channel campaign updates in real time across email, websites, and social media.
- Regulatory audit streamlining: Simplifies content tracking and audit processes, ensuring regulatory compliance.
Getting Started: Building a Unified Automation Model
Integrating content management, business logic, and intelligent orchestration within a low-code framework creates a unified automation model that delivers agility and ensures compliance. This approach empowers pharmaceutical organizations to optimize processes, enhance stakeholder engagement, and align with regulatory requirements, positioning them for a future-ready operational strategy.
How the Unified Model Works in Practice
Example Workflow: Regulatory-Compliant Content Delivery
- Content creation: Regulatory-compliant content is developed in VVPM and tagged for approval.
- Business rules execution: A business rules engine assesses the content's approval status and routes it to SFMC for distribution.
- End-to-end integration: Low-code platforms facilitate seamless data exchange across systems like VVPM and SFMC, with middleware such as Workato acting as the integration bridge.
Configuring Automation in Life Sciences for a Unified Model
To ensure compliance with regulations like GDPR while meeting business objectives, a unified automation model must leverage automated workflows, intelligent orchestration, and low-code/no-code frameworks. Here's how to configure it:
1. Centralized content factory
Integrating DAM systems with various outbound platforms streamlines content management and distribution by automating processes based on status changes in DAM documents. A well-executed content factory integration connects content management systems like Veeva Vault PromoMats (VVPM) with downstream platforms such as Salesforce Marketing Cloud (SFMC) or Sitecore. Middleware solutions ensure secure, scalable, and compliant data exchanges, enabling seamless content lifecycle management.

2. Middleware and business rules engine
A business rules engine enables organizations to enforce predefined logic across various systems, ensuring seamless data transformation, filtering, and redirection. By integrating business rules into workflow automation, companies can maintain consistent data management, improve operational efficiency, and drive compliance across interconnected platforms. Low-code/no-code platforms like Workato simplify the deployment and management of these automation processes, making rule enforcement scalable and adaptable.

Delivering Unified Automation: A Successful Case Study in Life Sciences Integration
A leading global pharmaceutical company based in Europe faced mounting challenges in managing promotional materials across multiple channels while adhering to strict regulatory requirements. Manual processes not only introduced compliance risks but also caused operational inefficiencies and delays.
The Challenge
The organization needed a solution to address several critical pain points:
- Fragmented content management: Disconnected systems caused inefficiencies, making it difficult to optimize processes and increasing the likelihood of errors.
- Compliance challenges: Non-compliant or outdated content often remained active, exposing the organization to regulatory breaches and potential penalties.
- Slow approval workflows: Lengthy and cumbersome approval processes delayed content publication, slowing time-to-market for critical initiatives.
- Metadata inconsistencies: Variations in metadata across platforms created integration challenges, impacting system interoperability and data accuracy.
- Limited content visibility: Stakeholders lacked a centralized view of content, making it difficult to track progress, status, or alignment across systems.
The Solution
To overcome these challenges, the company deployed a comprehensive integration solution with four key components:
- Connected ecosystem architecture: A unified platform was designed, integrating:
- Veeva Vault PromoMats for content creation and approval
- Workato for workflow automation
- Salesforce Marketing Cloud (SFMC) for digital engagement
- OCE CRM for enabling field force efficiency
- Intelligent workflow automation: Automated workflows were introduced to streamline the content lifecycle, including:
- Intelligent routing of content based on its approval status
- Automatic triggering of actions for content repurposing or retirement
- Real-time status synchronization: A robust synchronization mechanism ensured real-time updates across all integrated platforms.
- User-based triggers and automated notifications kept stakeholders informed and proactive.
- Consistent data synchronization minimized errors and improved operational accuracy.
- Business rule engine: An intelligent business rules engine was implemented to:
- Automate content distribution based on approval status
- Manage CLM content deployment seamlessly
- Enforce compliance requirements systematically
- Control content access and availability across platforms
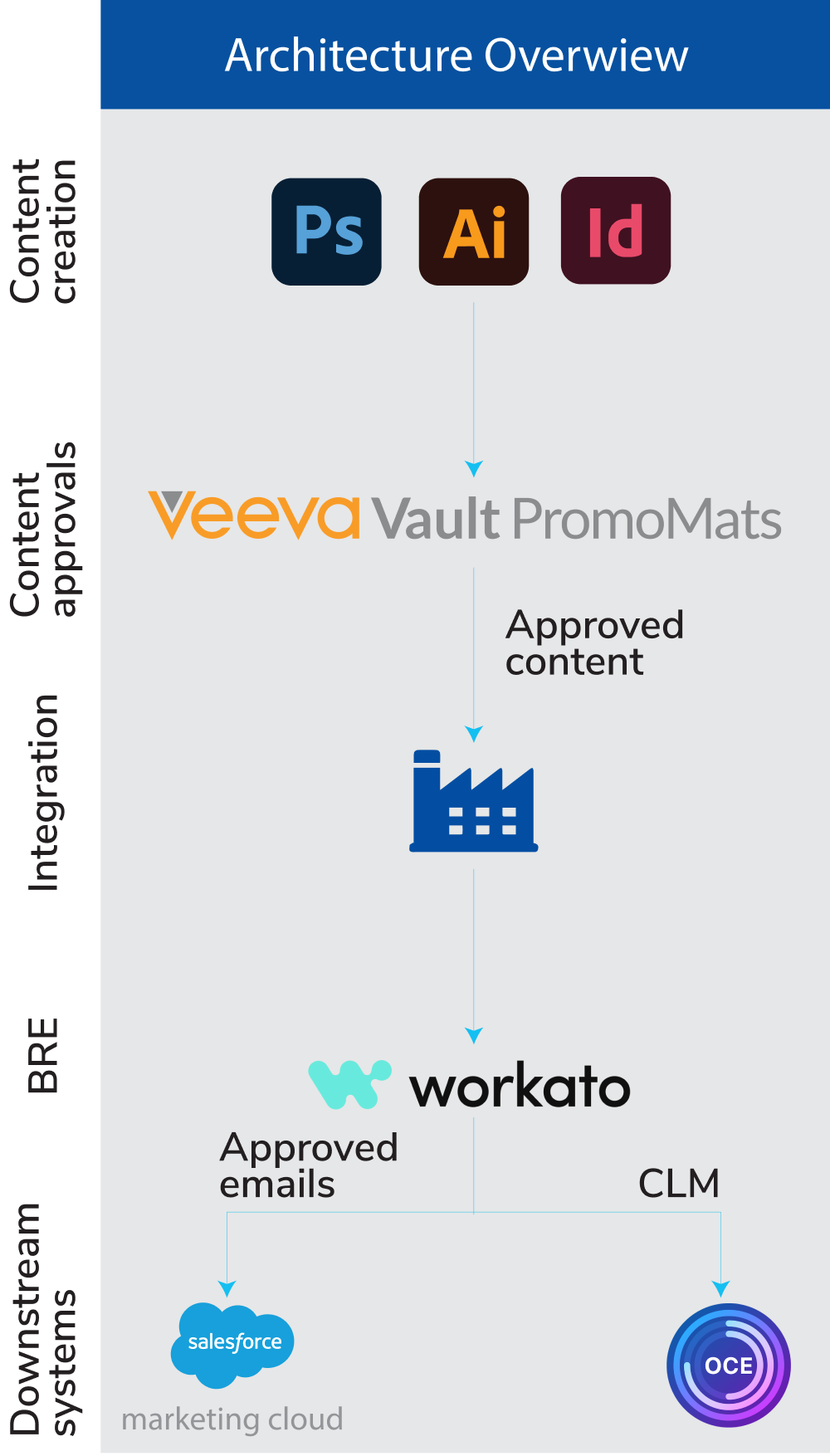
This integrated solution empowered the client to achieve significant operational efficiencies, mitigate compliance risks, and streamline the management of promotional materials. The unified automation model not only reduced manual intervention but also set a strong foundation for scalable, future-ready operations.
Unified Automation in Life Sciences Success
The future of automation in life sciences operations lies in adopting unified models that seamlessly integrate content factories, business rules engines, and low-code/no-code platforms. These technologies facilitate smooth data flow, ensure regulatory compliance, and enhance operational efficiency, delivering transformative results across global workflows.
By leveraging platforms like Veeva Vault PromoMats, Salesforce Marketing Cloud, and middleware solutions such as MuleSoft, organizations can automate complex processes, streamline approvals, and improve engagement with healthcare professionals and patients. Unified automation not only resolves current inefficiencies but also equips pharmaceutical companies to stay agile and thrive in an industry shaped by rapid innovation and evolving regulations.
Indegene offers decades of life science expertise, helping you successfully navigate today's connected commercialization landscape. We provide tailored solutions, combining people, processes, and platforms for long-term marketing success. Talk to us to learn more.
Let's Partner to Commercialize with Confidence


